Melanoma Clinical Trial Pipeline Accelerates as 150+ Pharma Companies Rigorously Develop Drugs for Market Entry | DelveInsight
The melanoma market is expected to witness sustained growth driven by continuous therapeutic innovation and wider patient access. Advances in immunotherapies and targeted treatments, along with earlier diagnosis and personalized care strategies, are significantly improving patient outcomes and reshaping disease management. Ongoing research and development, label expansions of existing drugs, and supportive regulatory frameworks are further propelling market momentum. With increasing healthcare investments and the entry of new biopharmaceutical players, the melanoma market is projected to remain highly competitive and innovation-driven in the coming years.
New York, USA, Oct. 14, 2025 (GLOBE NEWSWIRE) -- Melanoma Clinical Trial Pipeline Accelerates as 150+ Pharma Companies Rigorously Develop Drugs for Market Entry | DelveInsight
The melanoma market is expected to witness sustained growth driven by continuous therapeutic innovation and wider patient access. Advances in immunotherapies and targeted treatments, along with earlier diagnosis and personalized care strategies, are significantly improving patient outcomes and reshaping disease management. Ongoing research and development, label expansions of existing drugs, and supportive regulatory frameworks are further propelling market momentum. With increasing healthcare investments and the entry of new biopharmaceutical players, the melanoma market is projected to remain highly competitive and innovation-driven in the coming years.
DelveInsight’s 'Melanoma Pipeline Insight 2025' report provides comprehensive global coverage of pipeline melanoma therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the melanoma pipeline domain.
Key Takeaways from the Melanoma Pipeline Report
- DelveInsight’s melanoma pipeline report depicts a robust space with 150+ active players working to develop 170+ pipeline melanoma drugs.
- Key melanoma companies such as IO Biotech, Regeneron Pharmaceuticals, BioNTech SE, TILT Biotherapeutics LLC, Nykode Therapeutics, Amgen, Rapa Therapeutics, iOnctura, CJ Bioscience, Obsidian Therapeutics, IDEAYA Biosciences, Eikon Therapeutics, Microbiotica, Perspective Therapeutics, Pfizer, and others are evaluating new melanoma drugs to improve the treatment landscape.
- Promising pipeline melanoma therapies, such as IO102-IO103, Fianlimab, BNT111, TILT 123, VB10 NEO, ABP 206, RAPA-201, Roginolisib, CJRB-101, OBX-115, Darovasertib, EIK1001, MB097, [203Pb]VMT01, PF-08046049, and others, are in different phases of melanoma clinical trials.
- In September 2025, Microbiotica, announced that patient recruitment is complete in its advanced melanoma (MELODY-1) trial. This international trial has recruited 41 patients at clinical centres in the UK, France, Italy, and Spain. MELODY-1 is a Phase Ib study to evaluate the safety and tolerability of MB097 given in combination with pembrolizumab in patients with melanoma who demonstrate primary resistance to anti-PD-1 therapy.
- In September 2025, Servier, and IDEAYA Biosciences, Inc. announced an exclusive license agreement to bring darovasertib, a promising treatment for a rare eye cancer, to patients worldwide. Under the agreement, Servier obtains the regulatory and commercial rights for darovasertib in all territories outside the United States. IDEAYA retains its rights for darovasertib in the United States. Darovasertib, a potent and selective protein kinase C (PKC) inhibitor, is being developed to broadly address primary and metastatic uveal melanoma (UM).
- In July 2025, Replimune Group, Inc. announced that the US Food and Drug Administration (FDA) had issued a Complete Response Letter (CRL) regarding the Biologics License Application (BLA) for RP1 (vusolimogene oderparepvec) in combination with nivolumab for the treatment of advanced melanoma.
- In July 2025, Phio Pharmaceuticals Corp. announced it has entered into a comprehensive drug substance development services agreement with a US manufacturer. The company will provide analytical and process development and cGMP manufacture of Phio’s lead clinical development compound PH-762. This represents a critical next step in advancing Phio’s intratumoral program to treat cutaneous carcinomas. Phio is currently enrolling patients for the 5th and expected final cohort in its Phase Ib dose escalation study for cutaneous squamous cell carcinoma, Merkel cell carcinoma, and melanoma.
- In June 2025, Obsidian Therapeutics, Inc., announced initial Phase I safety and efficacy data from the Phase I/II Agni-01 multicenter study of OBX-115, a novel engineered tumor-derived autologous T cell immunotherapy (tumor-infiltrating lymphocyte [TIL] cell therapy) armored with pharmacologically regulatable membrane-bound IL15 (mbIL15), in patients with immune checkpoint inhibitor (ICI)-resistant advanced or metastatic melanoma.
- In June 2025, Phio Pharmaceuticals Corp. announced that the Safety Monitoring Committee (SMC) recommended dose escalation in its Phase Ib clinical trial designed to evaluate the safety and tolerability of intratumoral (IT) PH-762 in the treatment of Stages 1, 2, and 4 cutaneous squamous cell carcinoma (cSCC), Stage 4 melanoma, and Stage 4 Merkel cell carcinoma.
- In March 2025, IDEAYA Biosciences, Inc. announced that the US Food and Drug Administration (FDA) has granted Breakthrough Therapy designation (BTD) for darovasertib, a potential first-in-class protein kinase C (PKC) inhibitor, for the neoadjuvant treatment of adult patients with primary uveal melanoma (UM) for whom enucleation has been recommended.
- In March 2025, Perspective Therapeutics, Inc. announced that the first patient was dosed in a new cohort of a Phase I/IIa trial evaluating the safety of [212Pb]VMT01, a targeted alpha-particle therapy (TAT), in patients with histologically confirmed melanoma and positive melanocortin 1 receptor (MC1R) imaging scans.
- In October 2024, Microbiotica, announced that the first patient has been dosed in its advanced melanoma (MELODY-1) trial. This international trial is due to recruit up to 40 patients at clinical centres in the UK, France, Italy and Spain. Initial data readouts are expected by the end of 2025.
- In October 2024, Immatics N.V. announced updated Phase Ib clinical data on ACTengine® IMA203 TCR-T targeting PRAME in melanoma patients and provided an update on SUPRAME, the upcoming Phase III trial to evaluate IMA203 in metastatic melanoma patients.
Request a sample and discover the recent advances in melanoma drugs @ Melanoma Pipeline Report
The melanoma pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage melanoma drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the melanoma clinical trial landscape.
Melanoma Overview
Melanoma is a form of cancer that originates in melanocytes, the pigment-producing cells. While about 90% of melanomas arise in the skin, they can also develop in melanocytes located in the eye, internal organs, and mucosal surfaces lining the gastrointestinal, respiratory, and genitourinary tracts. It ranks as the third most common skin cancer after basal cell carcinoma and squamous cell carcinoma. Overall, it is the fifth most common cancer in men and the sixth in women. Melanoma has long been considered one of the most difficult cancers to treat pharmacologically, with slower therapeutic progress compared to many other cancer types.
Common sites of melanoma metastasis include the lymph nodes, lungs, liver, bones, and brain. Typical symptoms involve the appearance of a new or unusual growth or a change in an existing mole, such as increased size, color changes, darkening of the skin, an irregular border, a non-healing sore, or a spot that becomes painful, itchy, or tender. Melanomas can appear anywhere on the body.
To diagnose melanoma, a doctor conducts a skin exam and follows the ABCDE criteria. If skin cancer is suspected, a biopsy is performed. This may involve a punch biopsy, an excisional biopsy, or a shave biopsy. The tissue is then examined under a microscope to determine thickness, as thicker tumors carry a higher risk of spreading. Further tests may be conducted to identify metastatic melanoma or confirm the absence of a visible primary tumor. Additional diagnostic tools include sentinel lymph node biopsy, CT scans, MRI, PET scans, and blood tests, such as measuring lactate dehydrogenase (LDH) levels before treatment, as well as other blood chemistry and cell counts.
Treatment depends on the stage of melanoma and the patient’s general health. Early-stage melanoma is typically treated surgically, often offering a high chance of cure. Lymphadenectomy may be necessary if the cancer has spread to nearby lymph nodes, helping to prevent further dissemination. Metastasectomy can remove smaller metastatic lesions in various organs. Other treatment options include targeted therapy using drugs directed at specific cancer cells, radiation therapy, and immunotherapy, which has become a major focus among biopharmaceutical companies in managing melanoma.
Find out more about melanoma drugs @ Melanoma Treatment
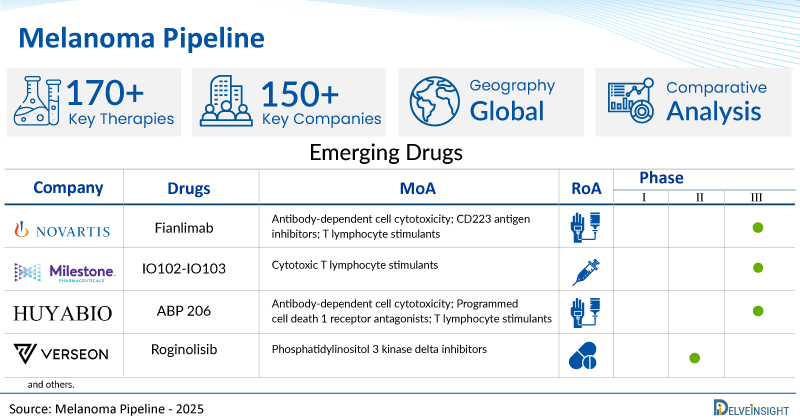
A snapshot of the Pipeline Melanoma Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| Fianlimab | Regeneron Pharmaceuticals | III | Antibody-dependent cell cytotoxicity; CD223 antigen inhibitors; T lymphocyte stimulants | Intravenous |
| IO102-IO103 | IO Biotech | III | Cytotoxic T lymphocyte stimulants | Subcutaneous |
| ABP 206 | Amgen | III | Antibody-dependent cell cytotoxicity; Programmed cell death 1 receptor antagonists; T lymphocyte stimulants | Intravenous |
| Roginolisib | iOnctura | II/III | Phosphatidylinositol 3 kinase delta inhibitors | Oral |
| BNT111 | BioNTech SE | II | Immunostimulants | Intravenous |
| CJRB-101 | CJ Bioscience | I/II | Macrophage modulators; Microbiome modulators | Oral |
| VB10 NEO | Nykode Therapeutics | I/II | Immunostimulants | Intramuscular |
| TILT 123 | TILT Biotherapeutics LLC | I | Cell death stimulants; Immunologic cytotoxicity; Interleukin 2 expression stimulants; Tumour necrosis factor alpha stimulants | Intravenous |
Learn more about the emerging melanoma therapies @ Melanoma Clinical Trials
Melanoma Therapeutics Assessment
The melanoma pipeline report proffers an integral view of the emerging melanoma therapies segmented by stage, product type, molecule type, route of administration, and mechanism of action.
Scope of the Melanoma Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Intravenous, Subcutaneous, Parenteral, Topical
- Therapeutics Assessment By Molecule Type: Monoclonal antibody, Peptides, Polymer, Small molecule, Gene therapy
- Therapeutics Assessment By Mechanism of Action: Cytotoxic T lymphocyte stimulants, Immunostimulants, Cell death stimulants, Immunologic cytotoxicity, Interleukin 2 expression stimulants, Tumour necrosis factor alpha stimulants, Lymphocyte replacements, Antibody-dependent cell cytotoxicity, CD223 antigen inhibitors, T lymphocyte stimulants, Programmed cell death 1 receptor antagonists, Phosphatidylinositol 3 kinase delta inhibitors, Macrophage modulators; Microbiome modulators, interleukin 2 expression stimulants; Tumour necrosis factor alpha stimulants
- Key Melanoma Companies: IO Biotech, Regeneron Pharmaceuticals, BioNTech SE, TILT Biotherapeutics LLC, Nykode Therapeutics, Amgen, Rapa Therapeutics, iOnctura, CJ Bioscience, Obsidian Therapeutics, IDEAYA Biosciences, Eikon Therapeutics, Microbiotica, Perspective Therapeutics, Pfizer, and others.
- Key Melanoma Pipeline Therapies: IO102-IO103, Fianlimab, BNT111, TILT 123, VB10 NEO, ABP 206, RAPA-201, Roginolisib, CJRB-101, OBX-115, Darovasertib, EIK1001, MB097, [203Pb]VMT01, PF-08046049, and others.
Dive deep into rich insights for new melanoma treatments, visit @ Melanoma Drugs
Table of Contents
| 1. | Melanoma Pipeline Report Introduction |
| 2. | Melanoma Pipeline Report Executive Summary |
| 3. | Melanoma Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Melanoma Clinical Trial Therapeutics |
| 6. | Melanoma Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Melanoma Pipeline: Late-Stage Products (Phase III) |
| 8. | Melanoma Pipeline: Mid-Stage Products (Phase II) |
| 9. | Melanoma Pipeline: Early-Stage Products (Phase I) |
| 10. | Melanoma Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Melanoma Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Melanoma Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the melanoma pipeline therapeutics, reach out @ Melanoma Therapeutics
Related Reports
Melanoma Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key melanoma companies, including Istari Oncology, Philogen, Georgiamune, Diakonos Oncology, Immatics Biotechnologies, Replimune, BMS, Ascentage Pharma, Erasca, Krystal Biotech, IDEAYA Biosciences, Novartis, HUYA Bioscience, Regeneron Pharmaceuticals, among others.
Ocular Melanoma Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key ocular melanoma companies, including Bristol Myers Squibb, Merck & Co., Inc., Novartis AG, Roche Holding AG, Pfizer Inc., AstraZeneca PLC, Eli Lilly and Company, Bayer AG, GlaxoSmithKline PLC, Amgen Inc., among others.
Uveal Melanoma Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key uveal melanoma companies, including Novartis Pharmaceuticals, Foghorn Therapeutics, TriSalus Life Sciences, Inc., Bristol Myers Squibb, Array BioPharma, Ono Pharmaceutical, AstraZeneca, Roche, IDEAYA Biosciences, Merck & Co, GlaxoSmithKline, Janssen, among others.
Metastatic Melanoma Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key metastatic melanoma companies, including Cancer Insight LLC, Elios Therapeutics LLC, Evaxion Biotech A/S, Merck Sharp & Dohme LLC, Novartis Pharmaceuticals, InxMed (Shanghai) Co. Ltd., Aivita Biomedical Inc., Hoffmann-La Roche, Idera Pharmaceuticals, Iovance Biotherapeutics Inc., Eisai Inc., Biocad, Myrexis Inc., Pain Therapeutics, Altor BioScience, among others.
Refractory Metastatic Melanoma Market
Refractory Metastatic Melanoma Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key refractory metastatic melanoma companies, including BioNTech SE, Y-mAbs Therapeutics, Seagen Inc., among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
